JOANNEUM RESEARCH – Breaking Down Barriers Towards Cheaper Healthcare
EARTO Innovation Awards 2017 – Impact Delivered Category
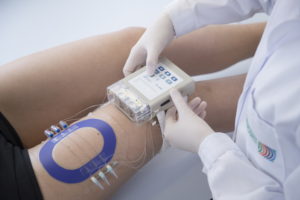 Generic drugs are an attractive, cost-effective alternative compared to original drugs. Their use enabled to save $3bn per week in 2010 in the US. But approval is only given to a generic drug which is bioequivalent to the original product: containing the same active substance to the site of action. Contrary to most generic drugs, bioequivalence testing for dermatological drugs, administered to the skin, cannot be carried out through blood testing. For such drugs, only clinical endpoint studies are accepted, which are time intensive, expensive, and associated with a high risk of failure.
Innovation: EARTO Member Joanneum Research in cooperation with the US FDA developed the first general applicable method to effectively assess bioequivalence of generic dermatological products. A patented technology (OFM) was used to develop a sampling set-up that can measure the active pharmaceutical ingredient of a drug directly in the skin, enabling to assess drug concentration.
Impact expected: This new approach may reduce generic drug development costs down to less than 20% of the current costs and clinical study time to 8 weeks instead of 1-2 years. This will enable pharmaceutical companies to develop new generic drugs more effectively, leading to more affordable medication and a significant reduction in health care costs.
Generic drugs are an attractive, cost-effective alternative compared to original drugs. Their use enabled to save $3bn per week in 2010 in the US. But approval is only given to a generic drug which is bioequivalent to the original product: containing the same active substance to the site of action. Contrary to most generic drugs, bioequivalence testing for dermatological drugs, administered to the skin, cannot be carried out through blood testing. For such drugs, only clinical endpoint studies are accepted, which are time intensive, expensive, and associated with a high risk of failure.
Innovation: EARTO Member Joanneum Research in cooperation with the US FDA developed the first general applicable method to effectively assess bioequivalence of generic dermatological products. A patented technology (OFM) was used to develop a sampling set-up that can measure the active pharmaceutical ingredient of a drug directly in the skin, enabling to assess drug concentration.
Impact expected: This new approach may reduce generic drug development costs down to less than 20% of the current costs and clinical study time to 8 weeks instead of 1-2 years. This will enable pharmaceutical companies to develop new generic drugs more effectively, leading to more affordable medication and a significant reduction in health care costs.
More information about this innovation
 Joanneum research is an Austrian RTO which focuses on application-oriented research and development projects to promote technology transfer into the economy.
www.joanneum.at
Joanneum research is an Austrian RTO which focuses on application-oriented research and development projects to promote technology transfer into the economy.
www.joanneum.at
< Previous Next >

